Introduction
Congenital hypotransferrinemia is a very rare inherited and life-threatening disorder (MIM#209300) characterized by systemic iron overload and anemia. The deficiency of serum transferrin leads to inadequate iron supply to erythroid bone marrow, resulting in microcytic hypochromic anemia. This triggers hepcidin downregulation, causing increased iron absorption and production of non-transferrin bound iron (NTBI), leading to iron overload in various organs and tissues. Currently, there is no targeted therapy for this disease.
Method
In an open-label phase II/III study, four children (age 0-7 years) and one adult (20 years) with congenital hypotransferrinaemia were treated with purified plasma-fractionated human apotransferrin for nearly 10 years. All patients received an initial intravenous dose of 75 mg/kg every 8 weeks for 6 months, followed by 75 mg/kg every 4 weeks for additional 6 months, and in the subsequent years apotransferrin was administered every 4 weeks but the dose was adjusted to 75-150 mg/kg based on clinical judgement of physician according to patient condition. Prior to the start of the study two patients already received replacement therapy, i.e., human apotransferrin or plasma. Serum transferrin and serum iron were determined before every infusion. Primary objective was to investigate the effect of apotransferrin treatment on anemia and iron overload. Hemoglobin, hematocrit, and erythrocytes as markers of anemia were measured every 8 weeks. Iron overload was monitored by measuring serum ferritin every 8 weeks and yearly iron quantification in liver and heart through MRI. Labile Plasma Iron (LPI) was measured before and after infusion (starting after 2 years of treatment). The need for additional treatment for anemia or iron overload was defined as a secondary outcome. Safety and clinical tolerance were evaluated by adverse event surveillance and laboratory measurements.
Results
Baseline serum transferrin levels were significantly below the normal range in all patients (<10-189 mg/L; normal range: 1800-3500 mg/L). Treatment with human apotransferrin increased serum transferrin and serum iron levels in all patients. Fifteen minutes after the first infusion, transferrin levels ranged from 1340-2415 mg/L, but declined to values just above baseline before the next infusion (dosing interval 8 weeks). During the study period serum transferrin concentrations were affected by dose adjustments; however, trough levels of transferrin remained below the normal range throughout the study (range 200-800 mg/L).
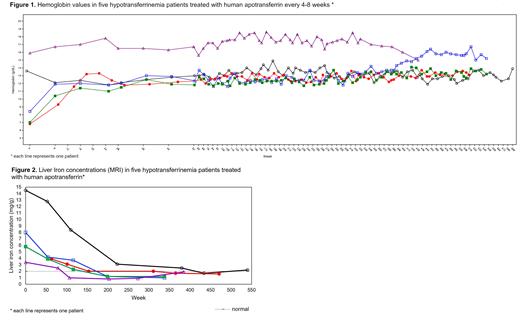
Despite subnormal trough levels, apotransferrin infusion led to rapid increase of hemoglobin (Figure 1), hematocrit, and erythrocyte levels up to normal values. The two patients who were already treated before the study, showed normal levels at baseline that were maintained. In all patients hematologic parameters remained stable throughout the study.
Ferritin levels were elevated at baseline and decreased to normal ranges in 1.2 to 7.3 years, with a maximum decrease ranging from 82% to 97% of baseline. MRI examinations revealed liver iron overload at baseline which normalized in all patients after apotransferrin treatment, in most cases after 3 to 4 years (Figure 2). No myocardial iron overload was observed at baseline and during the follow-up. Pre-infusion LPI was found in all five patients, however in three patients the values were undetectable on most occasions. Immediately after infusion with apotransferrin, in none of the patients LPI was present anymore.
Throughout the study no additional use of plasma/blood products or iron chelators was needed. Apotransferrin infusions were well tolerated. No serious adverse events were reported. No loss of clinical response to treatment was detected over time.
Conclusion
Treatment with human apotransferrin in patients with congenital hypotransferrinemia demonstrated good long-term clinical efficacy. Apotransferrin infusions increased hematopoiesis, reduced iron overload in organs, and eliminated the need for additional medication. The therapy was well-tolerated and showed good clinical safety. Human apotransferrin holds promise as the mainstay treatment option for congenital hypotransferrinemia.
Disclosures
Kleine Budde:Prothya Biosolutions: Current Employment. Díaz De Heredia:Biotest: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Other: travel expenses; Prothya Biosolutions: Research Funding. Mariani:Elma Research: Consultancy; IQVIA Solutions, Italy: Consultancy; Prothya Biosolutions: Research Funding. Piperno:Prothya Biosolutions: Research Funding. Moser:Prothya Biosolutions: Research Funding. Bodewes:Prothya Biosolutions: Current Employment. Isaila:Prothya Biosolutions: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal